Pharmaceutical Drugs
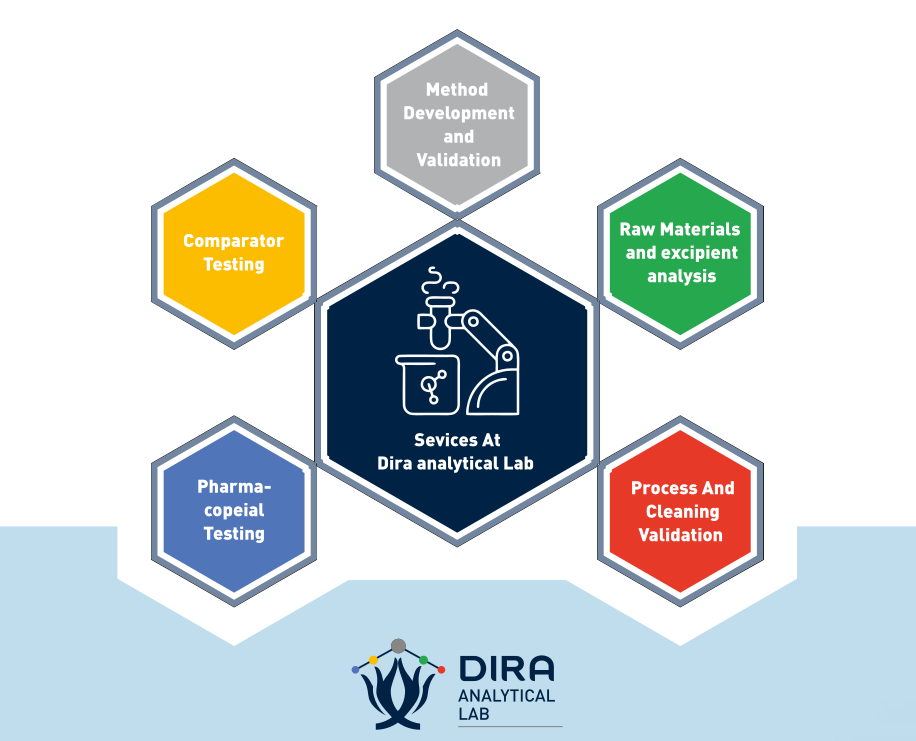
Laboratory Services Portfolio
Analytical Method Development and Validation
We offer analytical method developmst and validation services in compliance with current and national as well as international guidelines. The validations are done for all the parameters as per regulatory requirements

Pharmaceutical Drugs
- Toxicology Testing: Assess acute, sub-chronic, and chronic toxicity in animals to determine safe dosage levels.
- Pharmacokinetics (ADME): Evaluate Absorption, Distribution, Metabolism, and Excretion in preclinical and clinical phases.
- Efficacy Studies: Test drug effectiveness in targeted disease models (preclinical) and patient populations (clinical trials).
- Dose Response Studies: Identify the optimal therapeutic dose with minimal side effects.
- Clinical Trials (Phase I-IV): Progress through human studies to assess safety, efficacy, dosing, and post-market surveillance.
- Safety Profiling: Monitor short-term and long-term safety, including adverse effect detection and tolerability.
- Carcinogenicity and Genotoxicity: Investigate potential to cause cancer or genetic damage during long-term use.
- Stability Testing: Determine drug stability under various environmental conditions (temperature, humidity, and light).
- Microbiological Testing: Check for sterility, contamination, and microbial limits for quality assurance.
- Bioavailability and Bioequivalence: Measure drug absorption and compare generics with branded formulations.